 |
 |
Understanding the relationships between processes occurring on the seafloor and in the overlying water has been the focus of much of my research for the past 9 years. My Fulbright research will contribute to a large study in Norway investigating processe s controlling carbon cycling in sediments and the relationships between water and seafloor systems. Manipulative experiments in which I will add organic material (carbon) to bottom communities under controlled conditions will allow a direct, unconfounded test of the impact of food on these communities and the role of organisms in processing this carbon. The processes I will measure (respiration, sediment mixing) are important in determining the fate of organic material reaching the seafloor, which is one of the important factors necessary to predict the role of soft-sediment systems in global carbon cycling. Without this knowledge, we will be unable to predict the rate at which carbon dioxide from burning fossil fuels will accumulate in the atmosphere and possibly contribute to global warming.
Marine sediments play important roles in the marine ecosystem and the biosphere. They provide food and habitat for many marine organisms, some of which support commercial fisheries. More importantly from a global perspective, marine sediments also provide "ecosystem goods and services" (Erlich and Money 1983). Organic matter from primary production in the water column and contaminants scavenged by sinking particles accumulate in sediments where their fate is determined by physical, biological, and chemica l processes occurring at the sediment-water interface and within the sediment. Nutrients are regenerated and contaminants degraded by these processes. Under some conditions, carbon accumulates in coastal and shelf sediments and may be removed from the car bon cycle for millions of years, having a potential impact on global climate change. The economic value of services provided by coastal areas alone has recently been estimated to be on the order of $12,568 x 109 per year (Constanza et al. 1997), far in ex cess of the global GNP.
Nearly all marine benthic (bottom) communities below the photic zone (water depth with sufficient light for photosynthesis) are dependent on primary production by photosynthetic organisms in the water column for their energy. Consequently, the transfer of organic material between pelagic (water column) and benthic systems and the fate of this material upon reaching the bottom are central topics in biological oceanography (Fowler and Knauer 1986; Bruland et al. 1989; Smith and Kaufman 1999). A large portio n of the organic material reaching the benthos may be regenerated or mineralized (converted to carbon dioxide or other inorganic molecules), and sediment oxygen uptake rates, reflecting aerobic respiration, increase with increased input of food to the ben thos (Hargrave 1973; Davis 1975; Grebmeier et al. 1989; Grebmeier 1993; Pfannkuche and Thiel 1987; Smith et al. 1997).
The response of benthic organisms to food input is usually measured in either on- board incubation cores (Clough et al. 1997 and references therein) or in benthic lander (remote vehicle) chambers (e.g. Roe et al. 1997). In both cases, the area incubated i s typically small, usually less than 500 cm2 (Piepenburg et al. 1995). Consequently, incubation only measures the contributions of small organisms to carbon remineralization and misses the potentially important contribution of large, often mobile or deep dwelling, organisms. Recent work (Piepenburg et al. 1995; Piepenburg and Schmid 1996, 1997; Ambrose and Clough unpub. data) has demonstrated that epibenthic megafauna can account for up to 75% of carbon mineralization. Preliminary experiments in the Chukc hi Sea demonstrate that these organisms increase their respiration in response to deposition of fresh organic material (Ambrose and Clough unpub. data). The contribution of large benthic organisms to carbon mineralization and their response to organic deposition needs to be accounted for before an accurate picture of benthic carbon processing can be drawn.
 |
 |
The activity of benthic organisms at the sediment-water interface modifies the biogeochemical properties of this interface (Green et al. 1992), which can influence the strength of coupling between water column and benthic processes (Graf 1999) and the fat e of organic material reaching the bottom. One of the major modifications of the surface sediment created by biological activity is sediment mixing or bioturbation. The movement, burrow irrigation, and feeding activities of organisms strongly influence th e deposition, movement into the sediment, and early diagenesis (conversion to another form) of material reaching the seafloor (Graf 1989, 1999; Gerino et al. 1994; Levin et al. 1997). Rates of bioturbation are dependent on both the quantity (Clough et al. 1997) and quality (Dauwe et al. 1998) of organic mater reaching the sediment surface. As with studies of benthic mineralization discussed above, most empirical examinations of sediment mixing are conducted on a small scale (e.g. Clough et al. 1997). Larg e mobile (Ambrose 1993) and sedentary (Posey et al. 1991) organisms can significantly disturb the sediment surface while deep-dwelling taxa, often missed by traditional coring, can rapidly subduct freshly deposited material to depths of 10 cm or more int o the sediment (Levin et al. 1997). The activity of macrofauna and their response to organic material settling on the sediment surface must be understood before the fate of carbon reaching the seafloor can be understood.
The results of many studies examining the impact of organic deposition on shelf and deep-sea benthic communities and the fate of this material have been difficult to interpret because sampling has been spatially and/or temporally confounded. Few studies ( Graf 1989; Graf et al. 1982; Ambrose and Renaud 1997; Rysgaard et al. 1998; Smith and Kaufmann 1999) have followed the response of a benthic community at one location over a season of productivity or a period of deposition and even the results of these st udies can be hard to interpret. Experimental additions of phytodetritus (plant material) to sediment cores or cores containing individual animals have been used to circumvent some of the problems of associated with shipboard sampling. I recently used this approach in the Chukchi Sea and observed a rapid response by the entire benthic community and of individual taxa to experimental additions of ice algae. But the limitations of on-board experiments confined the variety and replication of experiments I cou ld conduct. A thorough examination of the response of benthic organisms to input of fresh organic material requires a yearlong field study and/or laboratory experiments where input of organic material is manipulated.
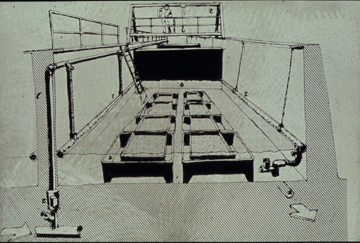
The overall objective of the proposed research is to quantitatively examine the impact of settled phytoplankton and ice algae on benthic community respiration and the respiration, bioturbation, and behavior of large, dominant taxa in the Oslofjord, Nor way. This work will be conducted at the University of Oslo's mesocosm facility (see figure 2) and is designed to complement a large, currently funded (European Community) project entitled "Key Coastal Processes in the Mesotrophic Skagerrak and the Oligotrophic North ern Aegean: A Comparative Study" (KEYCOP) (http://biologi.uio.no/keycop/) that is examining benthic-pelagic coupling. KEYCOP researchers will be conducting experiments in the mesocosm at the same time as my proposed research.
 |

|
The following specific questions will be addressed:
The proposed research contributes to a large study of the processes controlling coupling between water column and benthic systems with direct, unconfounded, tests of the impact of food on benthic communities. Water column processes are important in controlling the quality and quantity of organic material that reaches the seafloor, but processes on and in the sediment determine the fate of this material. In boreal and arctic systems and under productive, upwelling areas at lower latitudes, a large p ortion (over 90% in some high latitude fjords) of water column primary productivity reaches the benthos (references in Ambrose and Renaud 1995). Understanding the processes that determine the ultimate fate of the organic carbon reaching the seafloor is c ritical if we are to build accurate carbon cycling models for the marine ecosystem and the biosphere. Furthermore, the proposed experiments would represent the first simultaneous examination of the response of organic addition in three benthic communities and one of the few measurements of the impact of ice algae on benthic processes.
My Fulbright research will span 12 months, July 2000- July 2001. Work will begin in August with the collection of sediment and its transfer to the University of Oslo's mesocosm facility at Solbergstrand. Organic addition experiments will be conducted in t he late summer and early fall and experiments involving ice algae in the winter. Data will be analyzed and papers written in the spring and early summer, 2001.