- NITROGEN CYCLE, part 1:
Most of the Earth's nitrogen is in the atmosphere in the form of nitrogen gas, N2.
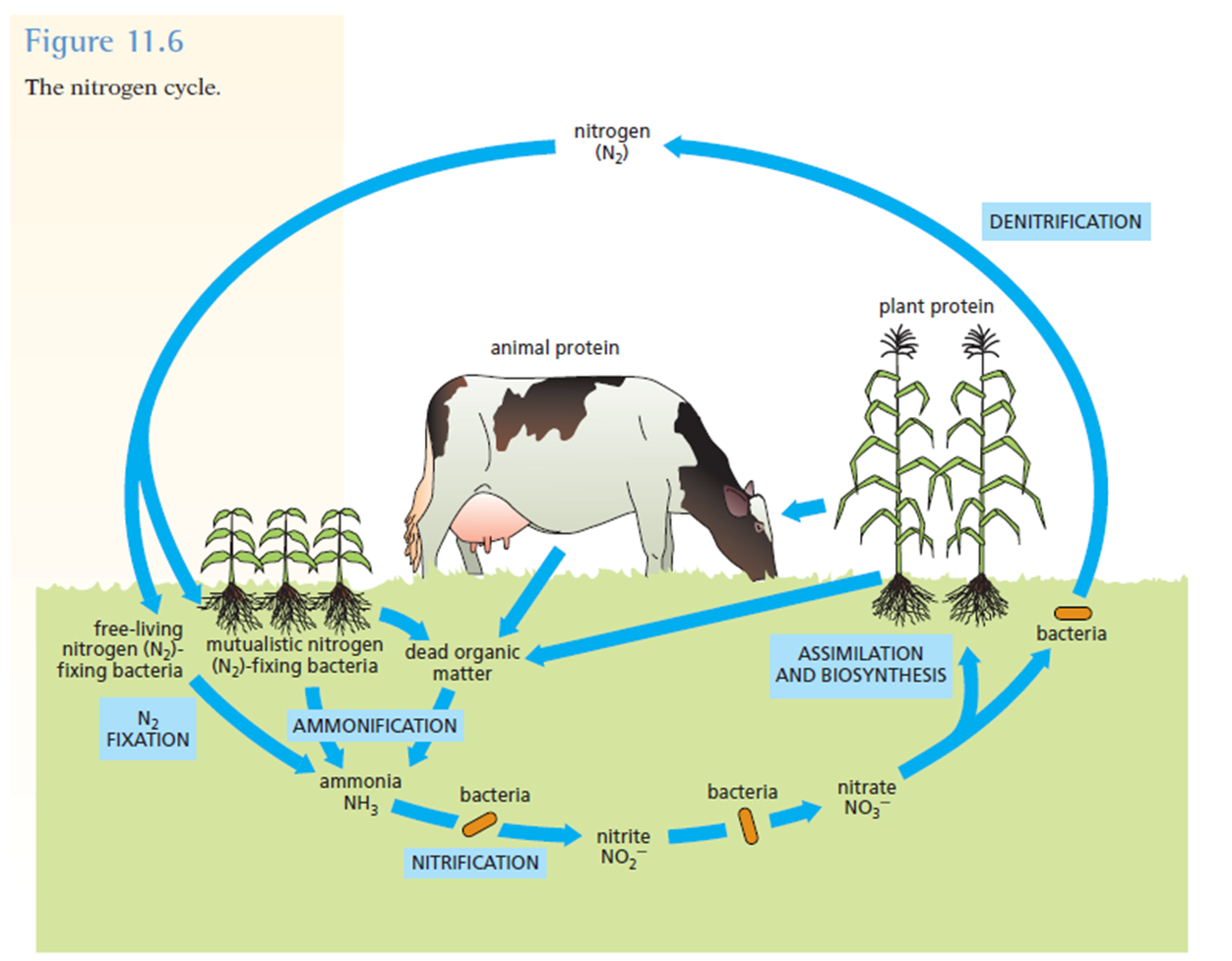
Nitrogen-fixing bacteria capture atmospheric nitrogen and "fix" it by converting it into nitrogen compounds.
Most of these compounds contain the ammonium ion (NH4+).
Some nitrogen-fixing bacteria live in the soil; others live in and around plant roots.
The plants take up the ammonium compounds and use the nitrogen to build up plant proteins.
Animals that eat the plants convert the plant proteins into animal proteins.
The dead bodies of plants, animals, and bacteria eventually decompose and release the nitrogen, mostly in the form of ammonia (NH3).
- NITROGEN CYCLE, part 2:
Ammonia (NH3) is converted into nitrites (NO2-) and then into nitrAtes (NO3-).
This process is called nitrification. It is carried out by different kinds of bacteria.
Nitrates, containing NO3-, are the form of nitrogen most abundant in soil and in rocks.
Most plants take up nitrates directly from the soil and convert the nitrogen into plant proteins and other organic nitrogen compounds.
Most nitrate compounds dissolve in water and are taken up by algae and aquatic plants.
Some nitrogen compounds are incorporated into rocks such as Chile saltpeter, which is mined as a source of chemical fertilizer.
A few bacteria, called denitrifying bacteria, break down nitrates and release nitrogen gas (N2) back to the atmosphere.
- Nitrogen fertilizers:
Nitrate compounds are commonly used as fertilizers to help plants grow more vigorously.
Nitrate fertilizers are soluble, so much of the fertilizer dissolves in groundwater and escapes as runoff, finding its way into streams, ponds, and lakes.
Aquatic algae thrive on dissolved nitrogen, so the runoff from fertilizers creates Algal blooms. Many algal blooms use up all the oxygen
in the water, killing many fish and other animals, and turning the water into a "dead zone".
|